Risk-Benefit analysis
- Risk Analysis is used for the assessment of the hazards associated with an industrial or commercial activity.
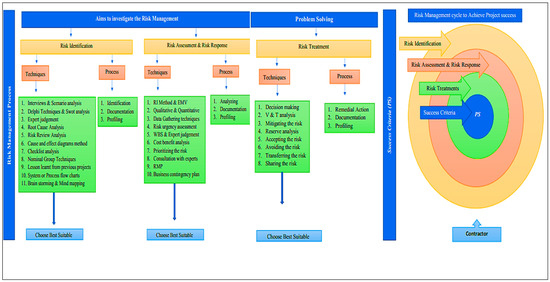
- Procedures: Hazard identification Consequences analysis Probability estimation f Risk - Analysis
- Risk analysis involves identifying the causes of unwanted hazardous events and estimating the consequences and likelihood of these events.
Full Answer
What are the different types of risk analysis?
- Systematic Risk – The overall impact of the market
- Unsystematic Risk – Asset-specific or company-specific uncertainty
- Political/Regulatory Risk – The impact of political decisions and changes in regulation
- Financial Risk – The capital structure of a company (degree of financial leverage or debt burden)
What is the best strategy in risk?
- Always concentrate your forces.
- Never be the first to attack head on.
- Start from Oceania! ...
- If a rival is obviously going for this, don't figth them. ...
- South America is also viable but you may get cornered without controlling any single continent. ...
- If a rival secures a continent, make a pact with anothe
What are the benefits vs. risks?
- Babies too young to receive vaccines
- Unvaccinated children and adults
- Pregnant women
- The elderly
- Individuals with weakened immune systems, such as those with asthma, chronic illness, or undergoing treatment for cancer
- Individuals who are allergic to vaccine components
What are the risk analysis techniques?
- Abstract. ...
- Introduction. ...
- Methods. ...
- Results. ...
- Discussion. ...
- Conclusion. ...
- Acknowledgements. ...
- Author information. ...
- Ethics declarations. ...
- Additional information. ...

What is meant by risk/benefit analysis?
Definition. Risk-benefit analysis is the comparison of the risk of a situation to its related benefits. For research that involves more than minimal risk of harm to the subjects, the investigator must assure that the amount of benefit clearly outweighs the amount of risk.
What is a risk-benefit analysis in ABA?
According to the Compliance Code, “a risk-benefit analysis is a deliberate evaluation of the potential risks (e.g., limitations, side effects, costs) and benefits (e.g., treatment outcomes, efficiency, savings) associated with a given intervention.
How do you conduct a risk/benefit analysis?
The IRB must:Identify the risks associated with the research, as distinguished from the risks of therapies the subjects would receive even if not participating in research;Determine that the risks will be minimized to the extent possible;Identify the probable benefits to be derived from the research;More items...•
What is the risk/benefit process?
Benefit-risk assessment is an integral part of FDA's regulatory review of marketing applications for new drugs and biologics. These assessments capture the Agency's evidence, uncertainties, and reasoning used to arrive at its final determination for specific regulatory decisions.
What is risk benefit analysis in professional ethics?
Risk-benefit analysis refers to the “systematic use of information to identify initiating events, causes, and consequences of these initiating events, and express risk (and benefit)” [4]. This, risk-benefit analysis refers to 1.) gathering of risk and benefit events, causes, and consequences; and 2.)
What is extinction ABA?
“Extinction” is a formal term, but it basically means our ABA therapists want to get to the bottom of the function or cause of a certain behavior and then terminate access to that function in order to extinguish the behavior.
Why is risk/benefit assessment important?
Robust risk benefit assessment is essential for such rich outdoor play to give children and adults freedom to play safely within a challenging environment. It is about weighing up the benefits of play against the level of risks involved.
What are limitations of risk/benefit analysis?
Limitation. The limitation is that the risks are a measure that is based on probabilities. So one can never be sure of a precise amount of the risk exposure at a given point of time. Also for the calculation ad the analysis of the risk no standard methods are there.
What is risk-benefit analysis quizlet?
What is a risk-benefit analysis? the comparison of the risk of a situation to its related benefits.
How can you reduce risk?
BLOGFive Steps to Reduce RiskStep One: Identify all of the potential risks. (Including the risk of non-action). ... Step Two: Probability and Impact. What is the likelihood that the risk will occur? ... Step Three: Mitigation strategies. ... Step Four: Monitoring. ... Step Five: Disaster planning.
Do you have to do a risk/benefit analysis before a product is placed on the market?
To summarize: ALWAYS carry out a Risk/Benefit analysis before a product is placed on the market, and include ALL risk items, regardless of their acceptability.
Is a risk/benefit analysis required for ISO 14971?
If a residual risk is acceptable, a risk/benefit analysis is not required. What often slips through the cracks is that the ISO 14971 is usually supplemented by additional requirements in each region. In particular, when the EU adopts a standard, additional information is added to it.
What is risk benefit analysis?
In a risk-benefit analysis, all risks and all benefits are combined in one and the same balance. This means that a disadvantage affecting one person can be fully compensated for by an advantage of the same size that affects some other person. In other words, interpersonal compensability of advantages and disadvantages is assumed [ Hansson, 2004 ].
How are risk and benefit assessments used?
Risk–benefit assessments on the basis of monetary values ( for example through shadow pricing) are frequently used in collective decision making. They are attractive, as they aim to show how to evaluate risks and benefits with respect to a common denominator, expressed in monetary units. The analyses are well-established, standardized to a large extent and ensure traceability of the arguments used. Risk–benefit analysis on the basis of cost assessments means to balance explicitly the monetary equivalents of risks and benefits of a variety of options thereby comparing and quantifying opportunities and risks. This comparison is made on the basis of a full monetization of all benefit and risk categories. A variety of concepts (opportunity costs, shadow prices, willingness to pay, price standard) are used to convert the benefits and expenditures (costs, organizational effort, costs of conflict, costs for decision-making etc.) of the different options for action into monetary units. Risks can then be evaluated by a simple calculation.
What are the components of risk management?
In accordance with the three major components ‘complexity, uncertainty, and ambiguity ,’ one can design different risk assessment and management strategies depending on the degree or intensity of each component ( Renn, 2008, 173ff ). For example, if a risk is characterised by high complexity, low uncertainty and low ambiguity, the main emphasis will be on scientific modeling and consensual methods for performing a most accurate assessment. If a risk is characterised by high uncertainty the main emphasis will be on reducing vulnerability of the system to cope even with surprises ( Bloesch et al., 2015 ). In a situation of high ambiguity a more discursive approach with stakeholder participation is asked for in order to find an acceptable way to reconcile conflicting values and world views ( Renn, 2015 ). More specifically, risk handling can pursue one of four strategies or a combination thereof. These strategies are summarized in Table 1 and described in the following paragraphs ( Renn, 2008, 182–183 ).
What can risk managers rely on?
Additionally, risk managers can rely on best practice and, in cases of low impact, on trial and error. Staff members of risk management agencies, industrial risk managers and experts in the field are the in ly groups that need to be involved for dealing with simple risks. •.
What is risk-risk comparison?
Traditional risk-risk comparisons (or risk-risk trade-offs), risk-benefit analysis and cost-effectiveness studies are the instruments of choice for finding the most appropriate risk reduction measures. Additionally, risk managers can rely on best practice and, in cases of low impact, on trial and error.
What is the basic principle of safety assessment?
No benefit–risk analysis is allowed under the Act. The basic principle of the safety assessment is confirmation of the novelty.
What makes risk-benefit analysis so attractive?
What makes risk–benefit analysis so attractive is that it offers a tool by which to orient risk evaluation to monetary values that can directly reflect societal benefits. Thus, costs for risks can be integrated into insurance premiums, while expected gains from opportunities can be integrated into shareholder prices or in the provision of venture capital. However, the integration of external effects (also referred to as externalities) and the associated need to value qualitative or quantitative changes of public goods present difficulties. Here so-called shadow prices that reflect people’s preferences that are not expressed on existing markets must be ascertained. While scientific approaches have been developed by which to perform this (so-called revealed or stated preference methods), these approaches yield substantially variable results, leading to ambiguity in many cases. A further difficulty is presented by the question of how to value costs and benefits that occur at different points in time. The procedure to follow is called discounting. While for market prices the usual interest rate on the market is sometimes adopted (notably in risk–benefit analyses from a private, that is, not societal perspective), it is difficult to justify the choice of a market-based discount rate for the monetization of environmental impacts. While it makes sense to discount gains that are only expected in the distant future, it is scarcely plausible to appraise the victim of a future damaging event as being less “valuable” than the victim of a present exposure. The above problems are particularly striking when dealing with risks to human health and ecosystems. For example, it is obvious that developing cancer in a later stage of life is preferred over a cancer incidence in the near future. In this case, discounting is plausible. However, if a risk kills 1000 people in the far future or totally different 100 people in the coming days there is no plausible reason for discounting the losses.
What is benefit risk analysis?
The benefit risk analysis is one of the key elements of the risk management process for medical devices and, more in general, a key element for any quality system. We have already been talking extensively of risk management activities, including ISO 14971:2019 and the related technical report ISO/TR 24971:2020 .
What is benefit risk determination?
According to Article II of the EU MDR, “benefit-risk determination” means the analysis of all assessments of benefit and risk of possible relevance for the use of the device for the intended purpose, when used in accordance with the intended purpose given by the manufacturer.
What is clinical benefit?
Clinical benefit is defined as the positive impact of a device on the health of an individual, expressed in terms of a meaningful, measurable, patient-relevant clinical outcome (s), including outcome (s) related to diagnosis, or a positive impact on patient management or public health. According to the EU MDR, the benefit-risk determination shall ...
What are the criteria used to evaluate individual risks?
In fact, the criteria used to evaluate individual risks usually include limits for the probability of occurrence of harm with a particular severity. The criteria used to evaluate the overall residual risk are often based on additional elements, such as the benefits of the intended use of the medical device.
Is it difficult to apply a rigorous approach to risk-benefit analysis?
For this reason, it is necessary to take in consideration some specific aspects that could help to simplify the analysis. For example:
Is benefit risk determination part of technical documentation?
According to the EU MDR, the benefit-risk determination shall be part of the technical documentation. Infact, Annex II clearly states that the technical documentation shall include :
What is the importance of risk-benefit analysis in clinical research?
One important aspect of the evaluation of innovative interventions is an adequate risk-benefit analysis. Interventions considered as clinical innovation should be subject to a risk-benefit profile evaluation according to the standards of medical practice, not research ( Levine, 1979, p. 22). According to Weijler and Miller (2004) and as stated by King and Churchill (2008), the risk-benefit analysis in clinical research is considerably distinct from the evaluation in medical practice. While therapeutic and nontherapeutic risks (that are not directly associated with therapeutic procedures) occur in the context of clinical research, all risks directly relate to the individual in the case of medical practice. In the case of research, nontherapeutic risks that occur in the context of nontherapeutic procedures and that are unrelated to the intended improvement of a patients' well-being procedures are balanced with potential therapeutic benefits to the research subjects and ought to be in reasonable relation to the knowledge gain and social benefit to society ( Weijler and Miller, 2004; Emanuel et al., 2000 ). 6 Hence, benefits to society are an additional factor in the assessment of research and the threshold for potential benefits to individuals might be considerably lower than in the case of medical practice ( King and Churchill, 2008 ).
When should risk benefit analysis be performed?
Risk benefit analysis should be performed prior to the administration of all CM, and the best combination of safety and diagnostic accuracy should be sought.
When we apply the aforementioned considerations on risk-benefit analyses to the concept of clinical innovation as new non?
When we apply the aforementioned considerations on risk-benefit analyses to the concept of clinical innovation as new nonvalidated practice, we argue that the ethical evaluation of a risk-benefit profile under clinical innovation should be in accordance with the ethical evaluation of medical practice. If the nonvalidated intervention is the only reasonable intervention for diseases with potentially life-threatening or strongly life-impa iring characteristics primarily designed to enhance the well-being of patients, high risks can be reasonably accepted.
What is the strongest evidence for therapeutic interventions?
The strongest evidence for therapeutic interventions is provided by the systematic review of randomized, triple-blind, placebo-controlled trials. Patient testimonials, case reports, and even expert opinions are not considered as strong evidence, because of the placebo effect and inherent biases. Most agencies, including the United Kingdom’s National Health Service (NHS), and the United States Preventive Services Task Force categorize evidence in four grades or levels. These levels are weighted; properly designed, RCTs have highest value. This is followed sequentially by nonrandomized controlled trials; well-designed cohort, case-control, multicentric studies; and multiple time series. The lowest weight is given to the opinions of authorities, clinical experience, descriptive studies, laboratory studies, and reports of expert committees. Depending on the strength of the evidence, and the balance between benefits and risks, evidence is categorized as good or fair. Any kind of conflicting evidence, where the risk-to-benefit balance cannot be assessed, is considered as insufficient, or poor-quality evidence. In 2000, an informal collaboration of people with an interest in reviewing health care grading systems formed a working group. This initiative is now known as the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group [11] ( www.gradeworkinggroup.org ). There are four general levels of quality of evidence according to GRADE guidelines: high, moderate, low, and very low quality of evidence.
What is risk analysis?
Risk analysis is a multi-step process aimed at mitigating the impact of risks on business operations. Leaders from different industries use risk analysis to ensure that all aspects of the business are protected from potential threats.
What is the difference between risk assessment and risk analysis?
Difference Between Risk Assessment and Risk Analysis. Risk assessment is just one component of risk analysis. The other components of risk analysis are risk management and risk communication. Risk management is the proactive control and evaluation of risks while risk communication is the exchange of information involving risks.
What is the difference between qualitative and quantitative risk analysis?
A key difference between qualitative and quantitative risk analysis is the type of risk each method results in. For qualitative risk analysis, this is projected risk, which is an estimation or guess of how the risk will manifest. Meanwhile, quantitative risk analysis deals with statistical risk.
What is the easiest method to analyze risk?
There are two main risk analysis methods. The easier and more convenient method is qualitative risk analysis. Qualitative risk analysis rates or scores risk based on perception of the severity and likelihood of its consequences. Quantitative risk analysis, on the other hand, calculates risk based on available data.
How to do root cause analysis?
How to Perform Root Cause Analysis. Step 1: Define the problem – In the context of risk analysis, a problem is an observable consequence of an unidentified risk or root cause. Step 2: Select a tool – 5 Whys, 8D, or DMAIC. 5 Whys involves asking the question “why” five times.
What is risk priority number?
The risk priority number is used to prioritize the potential failures that require additional planning. It’s a product of three factors: severity, occurrence, and detection.
What are some examples of risk analysis?
Here are risk analysis examples for three major industries: construction, transport & logistics, and manufacturing.
Risk a lot to Save a Lot
The IC will determine that there is a high chance of survivability and they have a strong chance of rescuing an occupant, protecting property or the environment. They will assign crews to do an aggressive fire attack and search while risking the health and potential death of a firefighter.
Risk a little to save a little
The IC will determine that there is a low to moderate chance of survivability or protecting the structure, property or environment. While there may be the potential for a patient to be rescued, the fire might need to be controlled before the firefighters enter the structure.
Risk nothing to save nothing
The IC will determine that there is no benefit to risking the firefighters’ life, safety and health when there are no signs of survivability or damage to the property/environment is too great. Significant fire involvement, extreme heat or building collapse would lead the IC to this decision.
Contributing factors into the Risk vs Benefit Analysis
Is the building occupied? At night, a house is expected to have people inside vs daytime when they might be at work/school
What is benefit risk analysis?
The benefit-risk analysis is not a choice. It is a requirement of ISO 14971. It helps a manufacturer establish if the benefits of a medical device outweigh its risks. However, before performing benefit-risk analysis, they must be able to answer the following questions:
What is magnitude of benefit?
Magnitude of the benefit: This is usually measured against a scale according to specific criteria.
What would happen if clinical evaluations were poorly designed and executed?
Quality of the clinical data: Poorly designed and executed clinical evaluations could render the clinical results unreliable and weaken the claim of the dominance of benefit over risks.
When deciding on the acceptability of the risks, manufacturers should identify the deficiencies in current therapies?
Additionally, when deciding on the acceptability of the risks, manufacturers should identify the deficiencies in current therapies to ascertain whether their device addresses a significant gap in the currently available healthcare. If the gap is not significant, then the device’s benefit-risk profile should be better than that of existing products and therapies.
What is clinical background?
Clinical background, including information on the clinical condition to be treated, managed or diagnosed, the prevalence of the condition, and the natural course of the condition.
Which quadrant of the risk analysis is best for a device?
Upper-left quadrant: If the analysed device delivers more benefit for the same or lower risk, the benefit to risk ratio is improved, and the benefit outweighs the risks.
Is it possible to balance the benefits and risks?
Thus, it’s often not easy or even possible to objectively balance the benefits against the risks. It’s like comparing apples with mandarins. However, the manufacturer must make a subjective decision to answer the following: